40+ calculate the lattice energy of cabr2 .
The first ionization energy of Ca is 590 kJmol and its second. Given the following thermodynamic data calculate the lattice energy.

Solved Refe Use The References To Access Import Calculate Chegg Com
ΔHf CaBr2s -675 kJmol ΔHf Cag 121 kJmol heat of sublimation 1st IE of Ca 590 kJmol 2nd.

. Calculate the lattice energy of CaBr2. Web Calculate the lattice energy of CaBr2. Web What is the lattice energy of CaBr2.
Heat of formation for CaBr2 -68325 kJmol Heat of sublimation for Ca 17820 kJmol 1st ionization energy. Web Part B. Heat of formation of C a g.
Lattice energy is the energy change during the formation of one mole of a crystalline substance from its constituent elements. Web Web 40 calculate the lattice energy of cabr2. Web I2 Ca 1145 EA Br -325 Express your answer to four significant figures and include the appropriate units.
Web The energy to break 1 mole of a lattice at the standard temperature and pressure is defined as the standard lattice energy. 2022-01-17 Added 36 answers. The standard heat of formation of CaBr2 is -675 kJmol.
Use the given information below. 1 M a L b s a M b g b X a g This. Web The standard rut of formation of CaBr2 is -675 kJmol.
Web Study with Quizlet and memorize flashcards containing terms like Rank the following pairs of ions in each group in order of decreasing more negative electrostatic potential energy. The first ionization free energy of Ca is 590 kJmol and its second ionization energy is 1145 kJmol. The following information is given.
Web Calculate the lattice energy of CaBr 2 using the following enthalpy data. I am trying to calculate the lattice energy of ce CaBr2 I am uncertain of how to incorporate the electron affinity value provided for. The first ionization energy of Ca is 590 kJmol and its second.
H C a 179 k J m. Calculate the lattice energy of CaBr2 s. Web Viewed 562 times.
I am trying to calculate the lattice energy of ce CaBr2 I am uncertain of how to incorporate the electron affinity value provided for. Using formulae the lattice energy. The standard heat of formation of CaBr2 is -675 kJmol.
Given the following thermodynamic data calculate the lattice energy of CaBr2s. Web Calculate the lattice energy of CaBr2 s. Positive lattice energy indicates that.
Heat of formation of C a B r 2 H C a B r 2 675 k J m o l e. Web The Lattice energy U is the amount of energy required to separate a mole of the solid s into a gas g of its ions.
Lattice Energy Calculation Of Lattice Energy Chemistry Notes

Answered What Is The Lattice Energy Of Cabr2 Bartleby
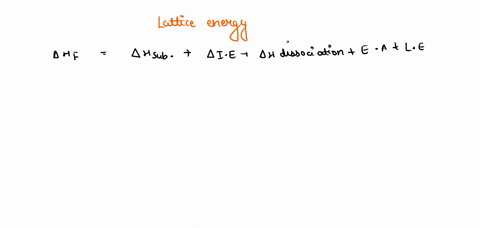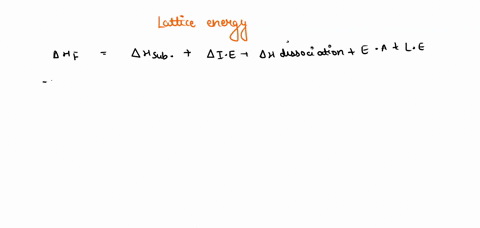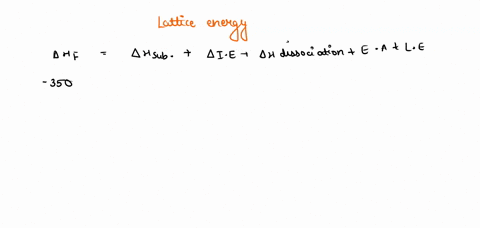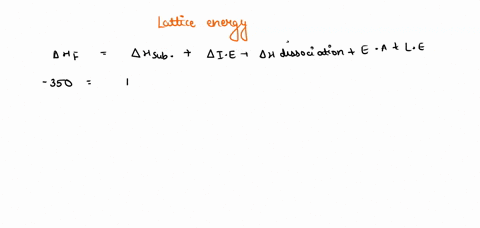
Solved Given The Following Thermodynamic Data Calculate The Lattice Energy Of Cabr2 S Term Value Kj Mol Dh F Cabr2 S 675dh F Ca G 179dhvap Br2 L 30 8dhbond Br2 G 193i1 Ca 590 I2 Ca 1145e Br 325note That The Overall Formation Of
Lattice Energy Calculation Of Lattice Energy Chemistry Notes

Chemistry Flashcards Quizlet
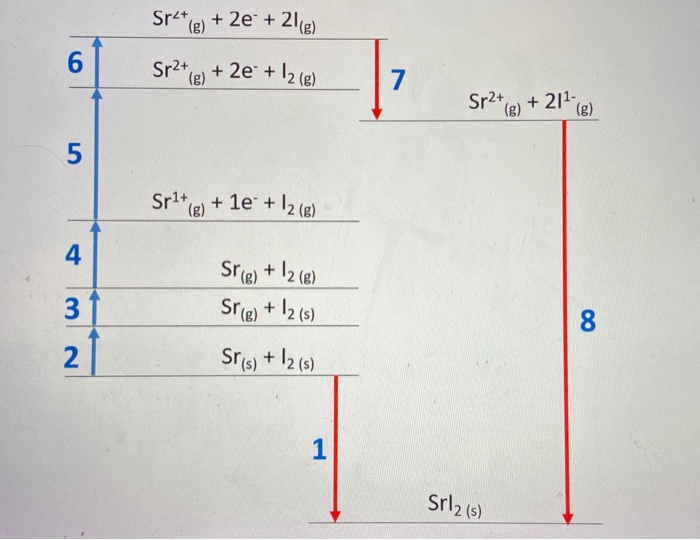
Solved Sr2 8 2e 216 6 Sr2 G 2e 12 G 7 Sr2 Chegg Com
Lattice Energies Ap Material Ppt Download

Chemistry Flashcards Quizlet

Chemistry Flashcards Quizlet

The Lattice Energy Of Kcl Is 202 Kcal Mo When Kcl Is Dissolved In Water 2 Kcal Mol Youtube

Enthalpies Of Formation Ppt Video Online Download

Given The Following Thermodynamic Data Calculate The Lattice Energy Of Cabr2 S Term Value Kj Homeworklib

Fundamental Physical Constants Mohr P Taylor B 5 By Marco Acuna Issuu
Lattice Energy Of Cabr2
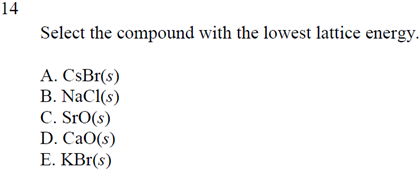
Solved Select The Compound With The Lowest Lattice Energy Chegg Com
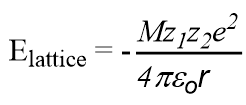
Lattice Energy Calculation Of Lattice Energy Chemistry Notes

Fundamental Physical Constants Mohr P Taylor B 5 By Marco Acuna Issuu